Contents of the cave
 The floor is strewn with angular rough-edged stones and soil to the back of the cave. At the end of the passage the floor consists of clay. The walls are partially sintered, mostly in the shaft.
The floor is strewn with angular rough-edged stones and soil to the back of the cave. At the end of the passage the floor consists of clay. The walls are partially sintered, mostly in the shaft.

| Cave Sediments Sediments, precipitates and minerals are included in the term cave sediments. Cave sediments can originate on the surface and be washed in, or can form in the cave itself. |
 |
|
There are three main groups of cave sediments: |
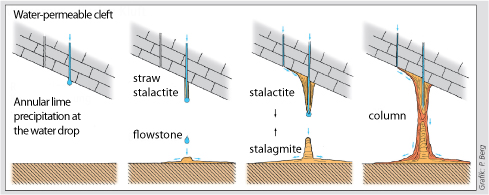
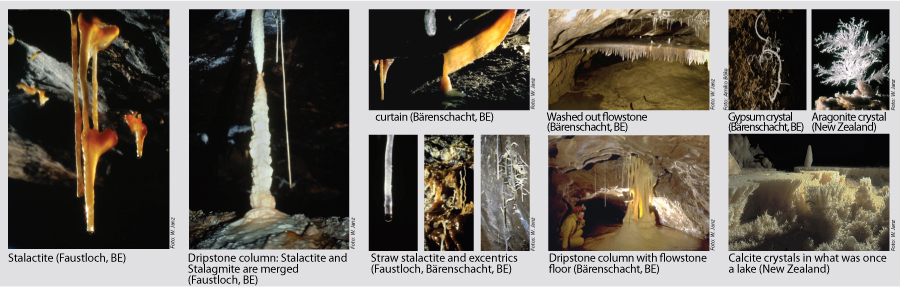
The speleothems refers to secondary mineral precipitates found in caves. The best known manifestations are the dripstones, i.e the downward hanging stalactites and the upward projecting stalagmites. Dripstones are formed when carbon dioxide is produced via root respiration and organic decay in the soil which is then diluted by rainwater seeping as carbonic acid through cracks in the rock, dissolving limestone in the process. When such water drips into a cave, carbon dioxide escapes in the form of gas, releasing the calcium carbonate which forms the dripstones. Evaporation is only a minor contributor. |
|
Detrital sediments (detrital means broken) are deposits from already existing rock or mineral, e.g. boulders, round pebbles from the stream, sand and clay.
Chemical sediments encompass all sorts of dripstones and calcite precipitates. Colours can change from white to yellow, or red and brown to black, depending on humus or mineral content. Other chemical sediments are mineral efflorescence on walls and deposits.
Biological sediments
Biological sediments contain animal bones, shells, guano (from bats), decaying plant material etc..
Ice in caves
Ice in caves is mostly present in caves where cold winter air has been trapped.


Chemistry of the Caves
The formation of the cave is based on penetrating carbonated water, formed from the CO2 of the soil air, which converts the hardly soluble calcium carbonate (15 mg/L) of the limestone into easily soluble calcium bicarbonate (1.5 g/l) and thus chemically dissolves it.
The reverse process also takes place and is called sintering.
The best known are the speleothems, e.g. the stalactites hanging down and the stalagmites growing upwards. These occur when water saturated with calcium bicarbonate enters the cave.
In the lower levels of carbon dioxide in the cave air an outgassing takes place, whereupon the water can no longer keep the entire dissolved calcium carbonate in solution. The excess crystallises and is deposited in the form of pure calcium carbonate. Evaporation is of secondary importance in this context.